Fertile soil contains a wide variety of microorganisms, so that no single method can be used for cultivating and enumerating all soil microorganisms.
- Factors influencing the size and variation in microbial population are :
- Differences in composition of soil.
- Differences in physical characteristics of soil.
- Differences in the agricultural practices.
- Variation in any one of these conditions causes variability in soil microflora.
Methods of studying soil microflora
There are various methods available for studying soil microflora such as- Direct Microscopic Method
- Agar Plate Technique
- Enrichment Culture Technique
- Buried Slide Method.
1]. Direct Microscopic Method :
- Soil suspension is prepared in aqueous solution of agar for good fixation.
- 0.1 ml of soil suspension is transferred to the ruled area on a microscopic slide and spread out uniformly.
- It is stained with 1% rose bengal dissolved in 5% aqueous solution of phenol.
- Bacteria appear deep pink or rose in colour. Minerals and inorganic consistituents do not stain. Some of the dead organic matter appears light pink but most of it stains either yellow or not at all. The slide is examined under a calibrated oil Immersion objective and the number of organisms per field counted.
- An average of atleast 25 field count is taken for the calculation of total number of bacteria per gram of soil.
Advantage :
- It is a rapid technique many sample can be examined in a short period.
Disadvantages :
- Microscopic observation is tedious.
- Bad smear preparation gives wrong results.
- Distinction between live cells and soil particles, as well as live cells and dead cells is difficult.
- Accurate determination by this method needs considerable experience.
2]. Agar Plate Technique :
- This method is used for isolation and enumeration of bacteria, yeast and molds present in soil.
- Suitable modifications are made to meet the cultural requirements of organisms to be enumerated.
- A weighed sample of soil is mixed with a known volume of sterile water in a screw-cap bottle or tube.
- The sample is shaken vigorously to separate organisms from the colloidal material surrounding soil particles.
- The coarse particles are allowed to settle down and a series of dilution are prepared from the suspension.
- Aliquote from each dilution is plated onto suitable agar medium.
- The plates are incubated at 25°C for 2-14 days and the results are expressed as CFU/gm of soil.
Advantages :
An exact picture of total viable cells can be obtained.Disadvantages :
- In a specific set of culture conditions only a part of the total microbial population can grow. Study of all types of microorganism cannot be done using a single medium and conditions for growth.
- Obligate anaerobes cannot grow under aerobic condition.
- Autotrophic bacteria cannot grow in organic media.
- Non-symbiotic N₂ fixers grow to a limited extent.
- Cellulose decomposers fail to grow on the commonly used media.
- Sulfate reducing organisms cannot grow in absence of sulfate in the medium.
- There is a great variety of molds found in soil. Number which appears on agar plate represents a small fraction of molds.
3]. Enrichment Culture :
- This method is useful for studying and obtaining a particular type of organism from a soil sample.
- Usually, a liquid or agar medium containing soil extract is used to enrich the rhizopods and ciliates type of protozoans.
- According to one technique, a short gram-negative, non-spore forming, bacterium such as Aerobacter sp. can be added to a minimal agar medium to cultivate protozoan, as it is easily digested by the protozoan fauna.
4). Buried Slide Method :
- The Buried Slide technique was introduced in the 1930s by Rossi and Cholodny for the direct microscopic study of soil microflora.
- This technique has also been described by Parkison et al. (1971).
- Clean, sterile glass-slides are inserted in situ by making slits in a soil by using a sterile knife. The soil may be dug out and used by filling it in a container in the laboratory.
- The soil is pressed around the slide for firm binding with glass-slide surface. It is then allowed to remain in place for 1-3 weeks.
- It is then removed and soil in cleaned from one surface. Gram staining or staining with phenolic aniline dyes is carried out after heat-fixation.
- Microscopic observation reveals a biofilm that represents soil microflora (colonies and single cells).
- It is possible that the microbial population may not be a representative of the soil.
Use of Winogradsky Column in Studying Microbial Diversity in Soil :
The Russian microbiologist Sergei Winogradsky (1856-1953), first developed a simple technique to demonstrate microbial communities and their interactions.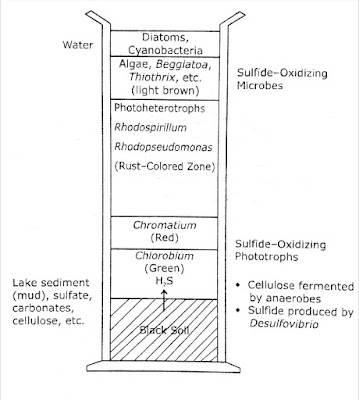 |
| The Winogradsky Column |
- A Winogradsky column consists of a core soil sample placed in a glass cylinder alongwith cellulosic waste, and overlaid with saline water or freshwater, which facilitates isolation of various sulfur metabolizing bacteria.
- The height of the column allows the development of aerobic zone at the surface and microaerophilic and anaerobic zones below the surface.
- The column is exposed to light so that various photosynthetic populations develop.
- The column consists of mud, CaSO₄, plant tissues (as source of carbohydrate) and water.
- It is exposed to daylight and incubated at room temperature.
The microbiological events can be summarised follows :
Initially, a variety of heterotrophic microorganisms present in soil oxidise various substrates depleting the oxygen supply and creating anaerobic conditions. Clostridium sp. can produce fermentation products under anaerobic conditions :
- Organic matter + O₂ ➞ organic acid + H₂ + CO₂ ↑ (cellulose,starch etc.)
- Organic acids + SO₄²⁻ ➞ H₂S + CO₂
Photosynthetic microorganisms such as purple and green sulfur bacteria Chromatium and Chlorobium use H₂S, as the electron donor to reduce CO₂ :
- CO₂ + H₂S ➞ (CH₂O)x + S.
- The purple sulfur bacteria are more tolerant to sulfide than green sulfur bacteria.
The aerobic sulfur metabolizing bacteria Thiobacillus develop in the upper portion of the column and oxidise reduced sulfur compounds like sulfides, elemental sulfur and sulphite to produce sulfate :
- Reduced sulfur compound SO₄²⁻ + accumulation of 'S°' (FeS, H₂S, S°, SO₃-)
The nonsulfur purple bacteria includes :
Rhodospirullum, Rhodopseudomonas, Rhodomicrobium, etc.
They are facultative phototrophs. They grow aerobically in the dark and anaerobically in the light. They can utilise sulfide at low levels, They use H₂ gas as electron donor in photosynthesis:
- CO₂ + H₂S ➞ (CH₂O)x + S°(in presence of light)
- CO₂ + 2H₂ ➞ (CH₂O)x + H₂O.
