BOD is the amount of oxygen utilized by microorganisms while stabilizing biologically decomposable organic matter in waste water under aerobic condition. BOD is a measure of organic pollutant load. Greater the oxidizable organic matter in water, the greater will be the BOD.
The BOD values are thus very useful in process designed to measure treatment plant efficiency and operation.
The origin of organic compounds are human excreta, vegetables, animal waste and industrial waste. The amount of organic matter present in water shows the amount of BOD. Large amount of sewage introduced into water body do not get diluted sufficiently. Microorganisms use up all the oxygen in the water to oxidize organic matter as a result of which water becomes useless for drinking and for other purposes.
The complete degradation of organic matter may take 20 to 30days. Simple organic matters like glucose are almost completely oxidized in 5 days while domestic sewage is oxidized to 65% in this period and complex organic compound gets oxidized only up to 40%.
The 20 to 30 days period is of least significance in practice. Therefore, the BOD test has been developed for 5 days at 20°C. BOD in general gives a qualitative index of organic substances which are degraded quickly in short period of time.
Principle of BOD :
Under alkaline conditions (by adding alkali-iodide azide), the manganese sulphate produces a white precipitate of manganese hydroxide, this reacts with the dissolved oxygen present in the sample to form a trivalent ion of manganese hydroxide giving brown precipitate.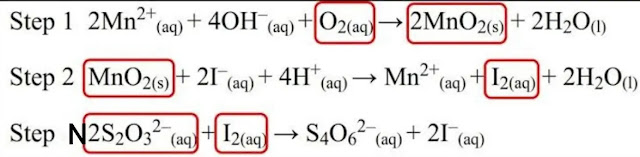 |
| BOD test chemical reactions |
Reagents and Apparatus:
- BOD bottle, BOD incubator, Burette, Pipette, Measuring cylinder.
- MnSO4 (Manganese sulphate) : Dissolve 48g of MnSO₄·3H₂O in 100ml of distilled water. Filter if necessary.
- Alkali- Iodide-Azide : Dissolve 50g of NaOH and 15g KI in 100ml distilled water. Add 1 g NaN3 dissolved in 4ml distilled water.
- Conc. H2SO4 :
- Starch Indicator (1%) : 1g of starch dissolve in 100ml distilled water.
- 0.025N Sodium thiosulphate : 1.24g of sodium thiosulphate in 200ml distilled water.
Procedure:
- Collect the waste water sample.
- Collect 300ml of waste water sample in BOD bottle using pipette without making air bubble.
- Add 2 ml of MnSO4 to the BOD bottle carefully by inserting the pipette just below the surface of water.
- Add 2ml of alkali-iodide-azide reagent in the same manner. Stopper the bottle immediately.
- Mix well by inverting the bottle 2-3 times, a brownish cloud will appear in the solution as an indicator of the presence of oxygen.
- Allow the precipitate to settle leaving approx. 150ml clear supernatant.
- Add 2ml of conc. H2SO4 carefully without forming air bubbles, mix the solution well to dissolve the precipitate.
- Take 200ml solution from this in Erlenmeyer flask and titrate against Na2S2O3 using starch as an indicator. (Note: Color change from bluish greenish to colorless)
- For blank use distilled water.
- Keep one bottle for determination of initial Dissolved oxygen (DO) level on 1st day and incubate 2nd bottle at 20°C for the determination of DO level on 5th day for waste water sample and Blank both.
Calculation:
Dissolved oxygen (DO):
DO = V1 X N X 8 X 1000/ Sample volumeWhere, V1= volume of titrant used; N= normality of titrant (0.025N);
Sample volume - 200ml; 8ml of Na2S2O3= 8 mg/L of DO
Calculation of BOD:
BOD= (D1-D5) - (C1-C5)Where, D1=DO in the Sample on 1stday; D5= DO in the Sample on 5th day;
C1=DO in the Blank on 1st day; D5= DO in the Blank on 5th day.
